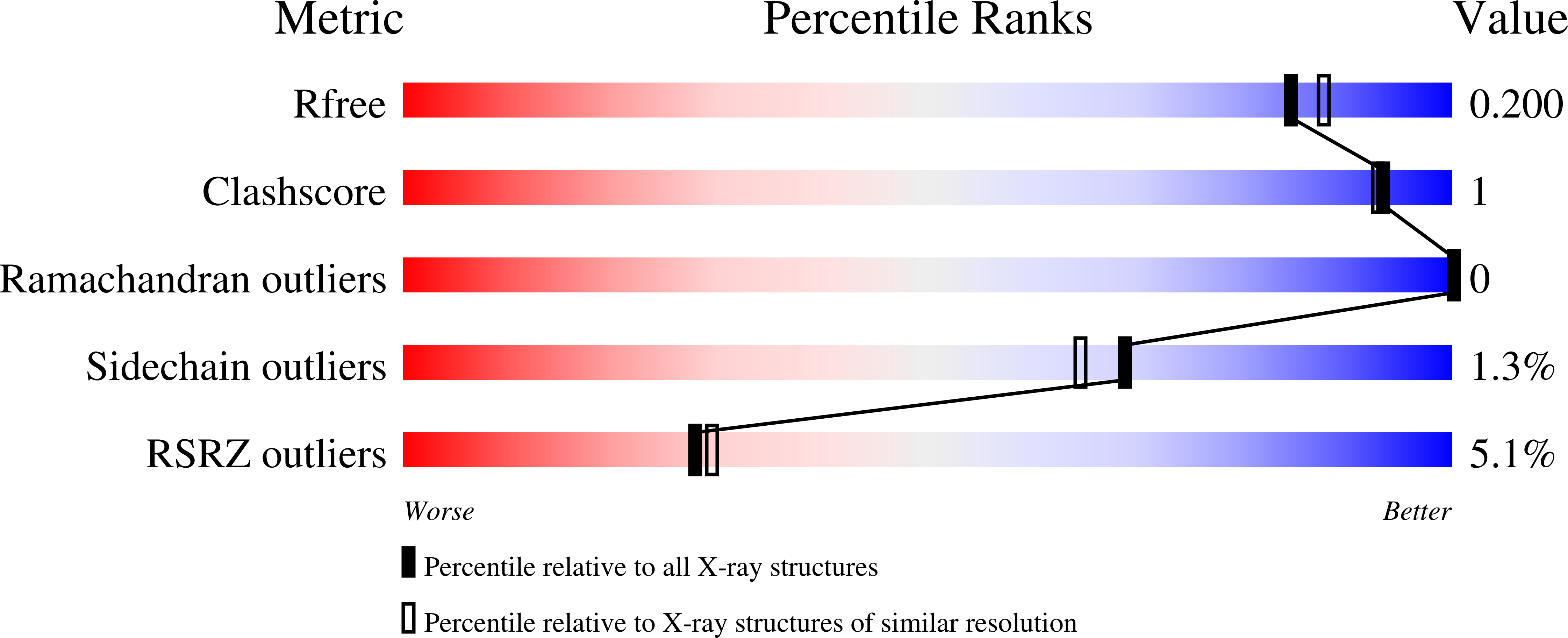
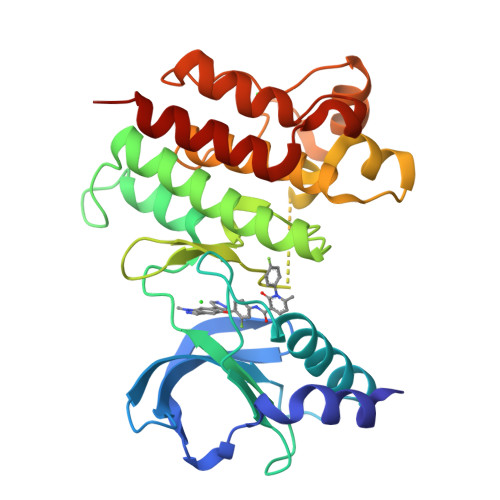
A-loop interactions in Mer tyrosine kinase give rise to inhibitors with two-step mechanism and long residence time of binding.
Pflug, A., Schimpl, M., Nissink, J.W.M., Overman, R.C., Rawlins, P.B., Truman, C., Underwood, E., Warwicker, J., Winter-Holt, J., McCoull, W.(2020) Biochem J 477: 4443-4452
- PubMed: 33119085
- DOI: https://doi.org/10.1042/BCJ20200735
- Primary Citation of Related Structures:
7AAX, 7AAY, 7AAZ, 7AB0, 7AB1, 7AB2 - PubMed Abstract:
The activation loop (A-loop) plays a key role in regulating the catalytic activity of protein kinases. Phosphorylation in this region enhances the phosphoryl transfer rate of the kinase domain and increases its affinity for ATP. Furthermore, the A-loop possesses autoinhibitory functions in some kinases, where it collapses onto the protein surface and blocks substrate binding when unphosphorylated. Due to its flexible nature, the A-loop is usually disordered and untraceable in kinase domain crystal structures. The resulting lack of structural information is regrettable as it impedes the design of drug A-loop contacts, which have proven favourable in multiple cases. Here, we characterize the binding with A-loop engagement between type 1.5 kinase inhibitor 'example 172' (EX172) and Mer tyrosine kinase (MerTK). With the help of crystal structures and binding kinetics, we portray how the recruitment of the A-loop elicits a two-step binding mechanism which results in a drug-target complex characterized by high affinity and long residence time. In addition, the type 1.5 compound possesses excellent kinome selectivity and a remarkable preference for the phosphorylated over the dephosphorylated form of MerTK. We discuss these unique characteristics in the context of known type 1 and type 2 inhibitors and highlight opportunities for future kinase inhibitor design.
Organizational Affiliation:
Structure, Biophysics and Fragment-Based Lead Generation, Discovery Sciences, R&D, AstraZeneca, Cambridge, U.K.